22 Jul Moderna Covid-19 Vaccine Trial to Launch at UCHealth
The phase 3 trial of Moderna’s mRNA vaccine candidate for COVID-19 will enroll 1,000 at University of Colorado Hospital, 30,000 nationwide. We curated this article by Ted Neff from UCHealth Today.

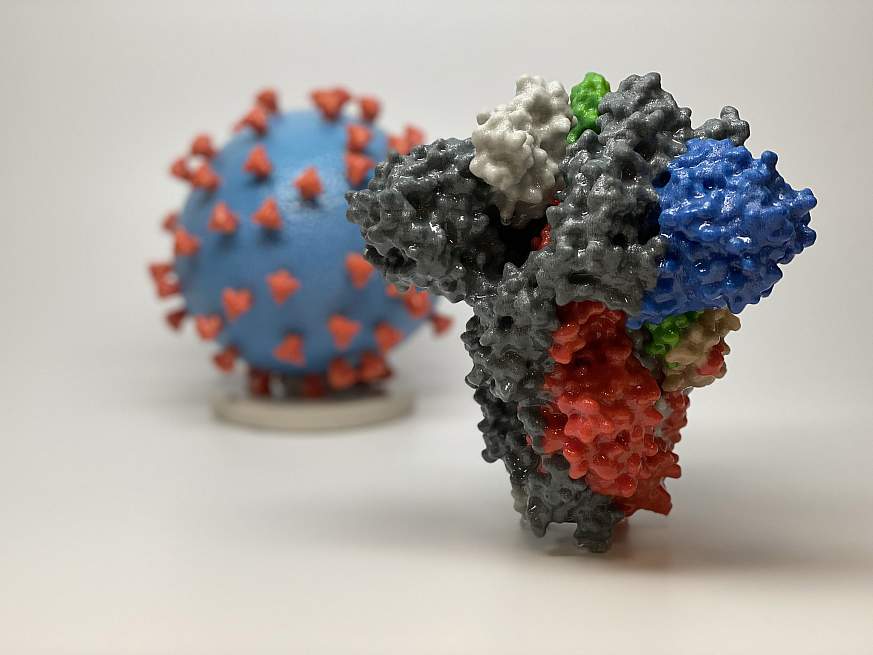
The Moderna coronavirus vaccine trial in Colorado is due to start later this summer. This image shows 3D prints of a spike protein (in the foreground) and a particle (background) of the virus that causes the new coronavirus. The spike protein enables the virus to enter and infect human cells, and it’s the target of many vaccine candidates, including Moderna’s vaccine. Photo courtesy of the National Institutes of Health.
UCHealth University of Colorado Hospital will soon enroll patients in the first large-scale U.S. clinical trial of a coronavirus vaccine.
The phase 3 study of Moderna’s experimental vaccine will include 1,000 people at University of Colorado Hospital on the Anschutz Medical Campus. In addition, another 30,000 patients nationwide will participate in the trial, which is slated to start this summer, says Dr. Thomas Campbell, a University of Colorado School of Medicine and UCHealth virologist and infectious-disease specialist.
Campbell’s team will internally recruit UCHealth patients, staff and providers based on a hierarchy of criteria, he says. Those in high-risk occupations such as essential workers in crowded facilities, people living in residential facilities and high-risk health care workers will be first up, Campbell says. People at risk of becoming seriously ill with COVID-19, including seniors and those with medical problems such as diabetes, obesity, heart disease, lung disease, and chronic kidney disease, will also be a priority, he says.
The UCHealth team will also invite individuals in higher-risk groups to participate in the study including Black, Indigenous and Hispanic patients.
After identifying possible trial participants, representatives from UCHealth will contact the patients and staff members through My Health Connection to invite them to participate, if interested.
“The emphasis is on demonstrating the efficacy of the vaccine in people who are at most risk of getting COVID-19,” Campbell said.
The phases of Moderna coronavirus vaccine trials
The trial will involve an initial injection and a booster 28 days later. It’s not a challenge trial: that is, patients will not be exposed intentionally to the virus that causes COVID-19. The huge number of participants, though, will allow the study’s leaders to compare the number and severity of coronavirus infections among those who got the vaccine with those who did not. Campbell says his team intends to move quickly and have all 1,000 patients injected with a first dose within eight weeks – and many would have received the booster by then also.
The study will follow patients for two years. That doesn’t mean a commercial vaccine must wait until 2022. In a best-case scenario, compelling preliminary results could bring speedy approval from the U.S. Food and Drug Administration (FDA). Were that to happen, widespread vaccination of the general public could roll out by next spring, Campbell says.
The FDA requires three phases of human clinical trials before it approves a drug (that’s in addition to having shown that the drug works in petri dishes, mice, and other animals). Phase 1 involves tens of people to make sure the drug is safe. Phase 2 generally involves hundreds of people and focuses on safety as well as what doses seem to work best. Phase 3 typically involves thousands of people and is used to prove safety and effectiveness.
Clinical trials normally proceed in sequential order over several years. These are not normal times. The phase 3 trial is launching with Moderna’s phase 1 trial data soon to be published and the phase 2 trial having just launched in late May. But the initial findings have been promising enough – and the desperation for a means of attaining COVID-19 herd immunity without swamping hospitals great enough – that the pace of science is being pushed to its very limits.
And watch this video about vaccine testing at UCHealth.


Sorry, the comment form is closed at this time.